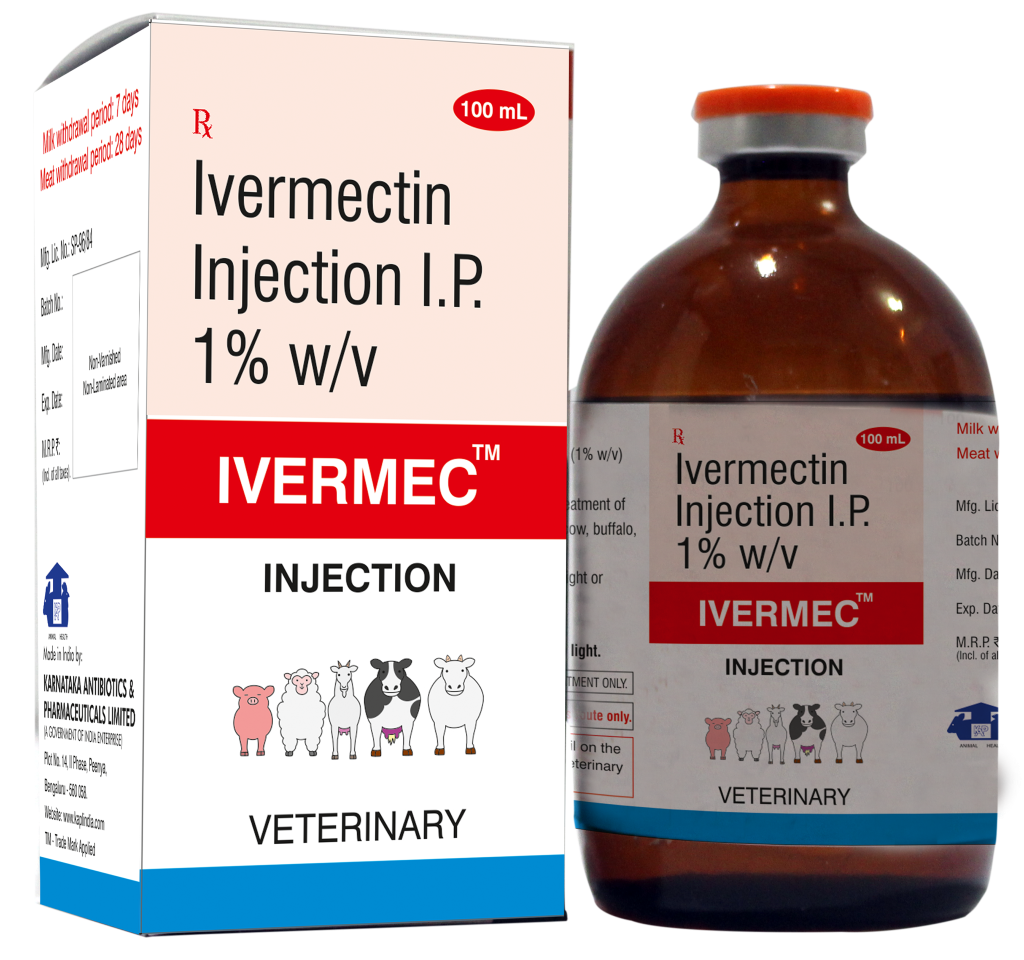
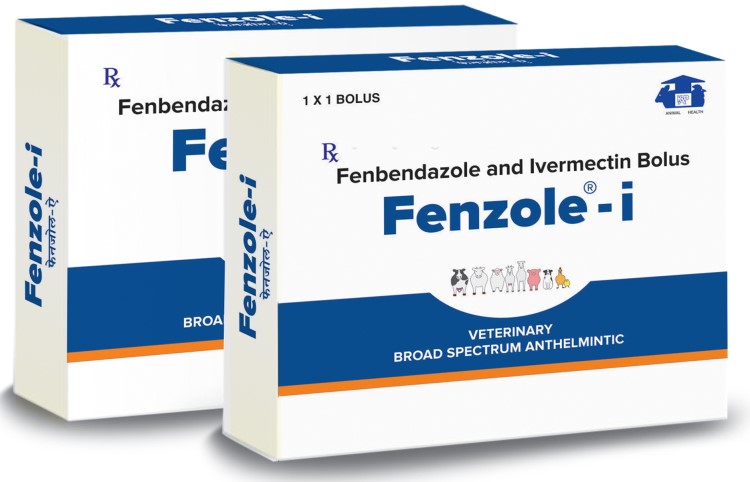
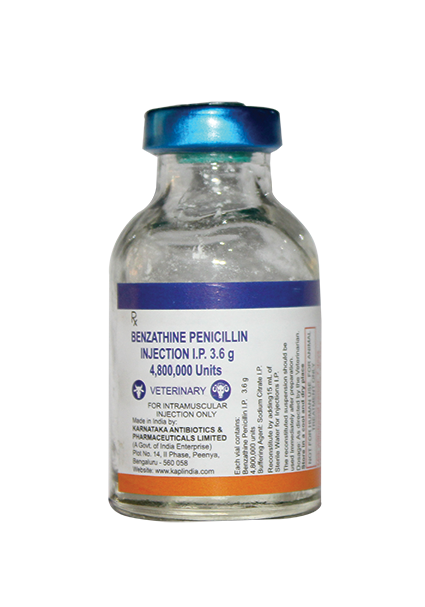
KARNATAKA ANTIBIOTICS & PHARMACEUTICALS LIMITED
[ A GOVERNMENT OF INDIA ENTERPRISE]
Registered & Corporate Office: Arka The Business Centre
Plot No. 37, Site No. 34/4,NTTF Main Road, 2nd Phase, Peenya Industrial Area,
Bengaluru – 560 058 Phone No.080-23571590
Fax. : 091(080)23371350. Website : www.kaplindia.com
************************************************************************************************************************************************************