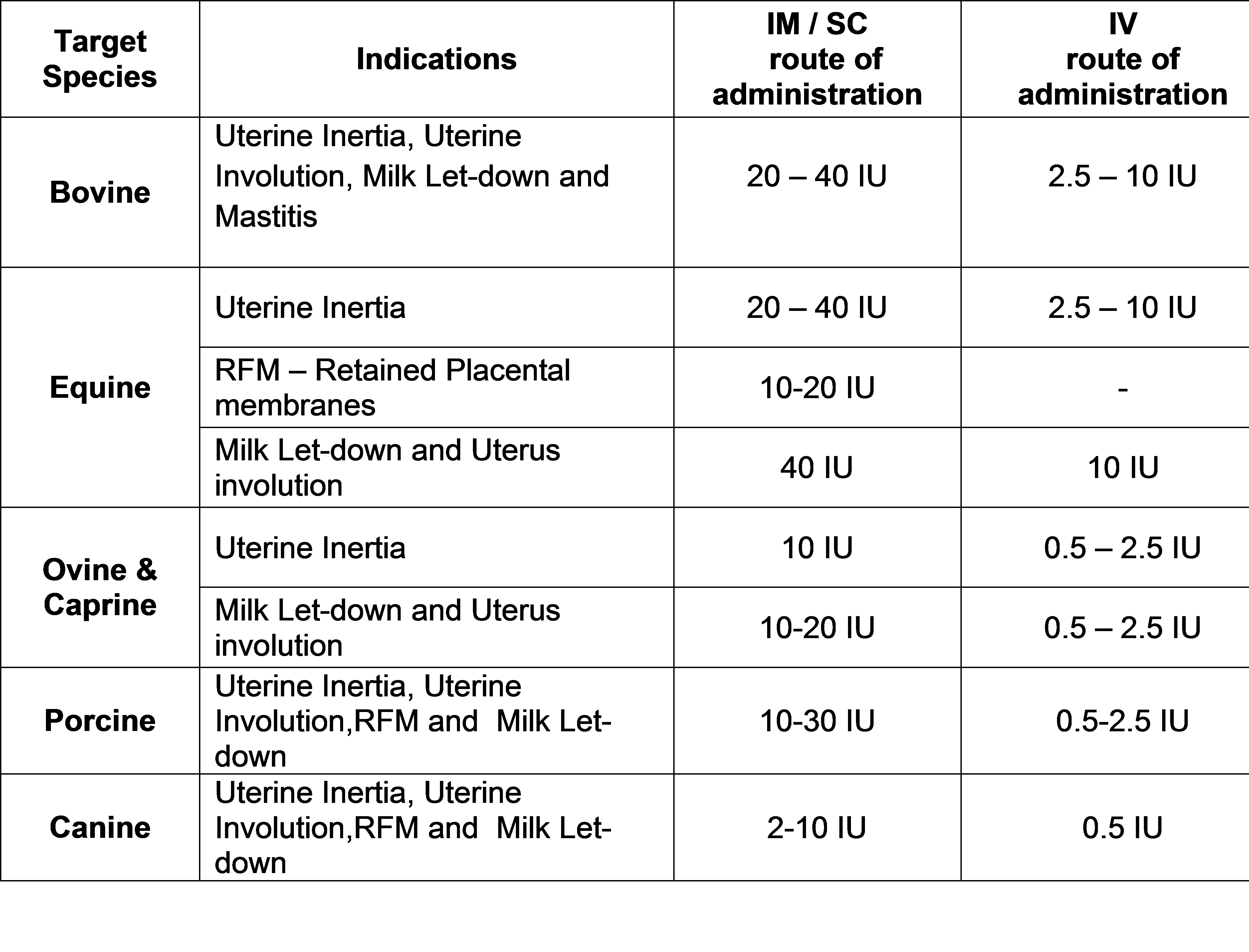A step above the conventional management of Acid Peptic Diseases
D E S C R I P T I O N / M O D E O F A C T I O N
Toprazol (Pantoprazole) is a pyridyl methylsulfinyl benzimidazole, which causes irreversible inhibition of proton pump (H+ K+ -AT Pase) function. Pantoprazole after absorption from the upper intestine enters the blood stream. From here it enters the parietal cell, crosses the cell membrane and gets concentrated in the canaliculi. In the canaliculi, because of the strong acidic condition it gets converted to sulphenamide derivatives. The sulphinamide derivatives of pantoprazole inactivate the sulfhydryl group of H+ K+Adenosine Tri-Phosphatase ( A T Pase), which in turn catalyses the final step of the gastric acid secretion pathway, thus inhibiting both centrally and peripherally mediated gastric acid secretion. It is rapidly activated under strongly acidic conditions. This pH dependent activation profile underlines the in-vitro selectivity of pantoprazole against H+K+ AT Pase compared with Omeprazole. Pantoprazole is rapidly absorbed after oral administration with peak plasma concentration of 1.1 to 3.1 mg / L (Cmax) occuring 2 to 4 hours (Tmax) after ingestion of an enteric coated 40 mg tablet. The drug is subject to low hepatic extraction, displaying an estimated absolute bioavailability of 77%. Elimination half-life of Pantoprazole is 0.9 to 9 hours. However inhibition of acid secretion, once accomplished, persists long after the drug has been cleared from the circulation and has a plasma protein binding of 98%. Pantoprazole undergoes extensive hepatic metabolism via cytochrome p 450 mediated oxidation followed by sulphate conjugation. Elimination is predominantly renal with 80% of an oral or intravenous dose being excreted as urinary metabolites, the remainder is excreted in the faeces and originates primarily from biliary secretion.
C O M P O S I T I O N
|
I N D I C AT I O N S
|
D O S A G E
It is given orally in a dose of 40 mg once daily before breakfast. Dosage adjustment is not required in the elderly and in patients with renal impairment or in those with hepatic impairment.
S I D E E F F E C T S
On short term administration, the most common adverse reported with the drug include:
Diarrhoea (1.5%)
Diziness (0.7%)<
Pruritis (0.06%)Skin rash (0.4%)<
These are generally mild or of moderate intensity, rarely necessitating treatment withdrawal.
P R E S E N TAT I O N & PACK
Toprazol tablet is available in blister pack of 10 x 10’s.